The Holy Grail of catalysis has finally been found! It was only possible to convert methane to methanol with the help of light.
international research teamLed by scientists at the University of Manchester, they have developed a fast and cheap way to convert methane (gas) into methanol (liquid). All this under standard pressure and temperature conditions.
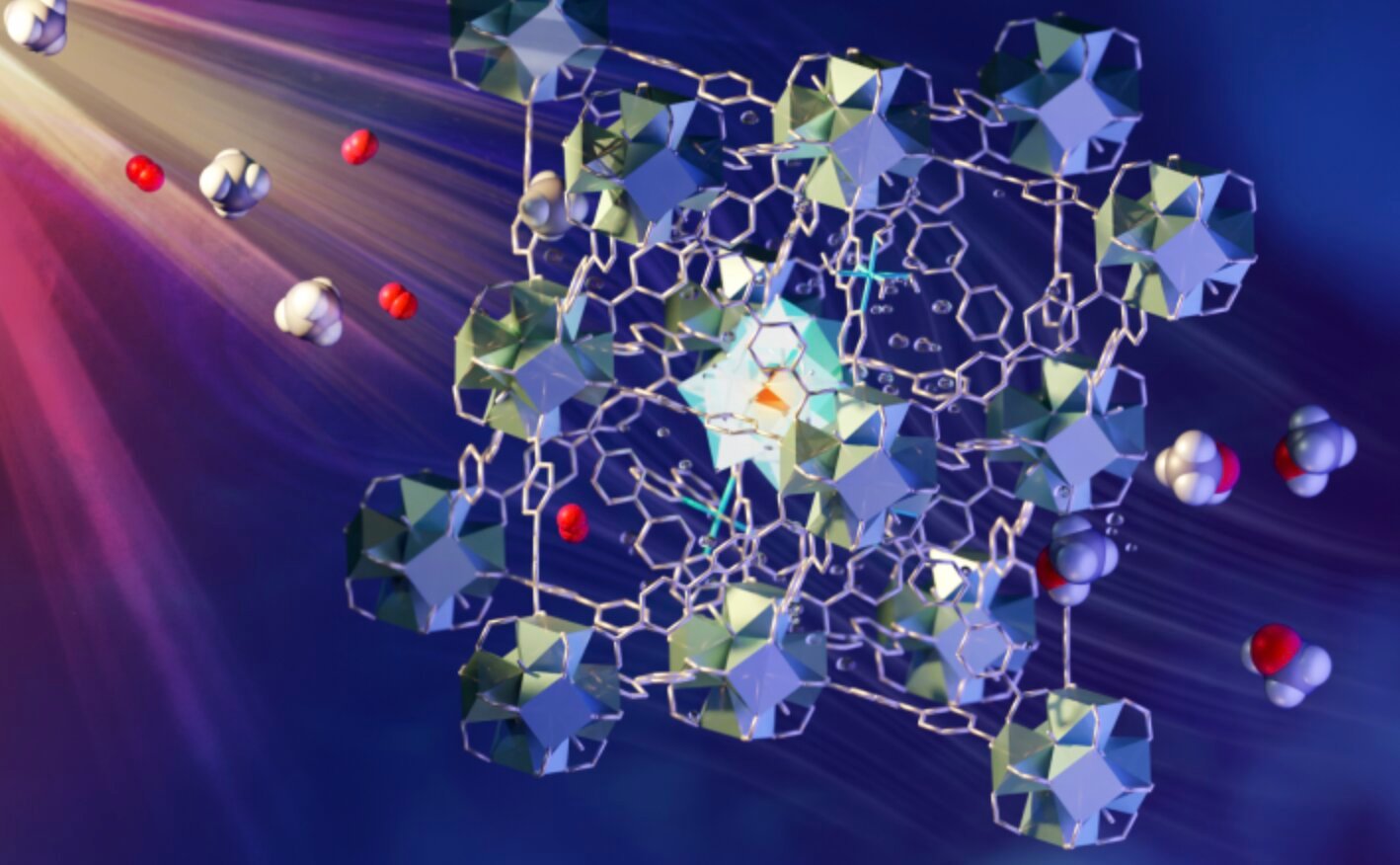
To see how the process itself works, the scientists used neutron scattering on the VISION instrument at the Spallation Neutron Source at Oak Ridge National Laboratory. This method involves the continuous flow of methane/oxygen saturated water through the MOF catalyst. It is porous and contains different components, each of which plays a role in absorbing light, carrying electrons, and activating and incorporating methane and oxygen. Such a process is widely seen as the “holy grail of catalysis” and is an area of interest for research supported by the US Department of Energy. The details of the discovery are described in nature materials.
How to convert methane to methanol?
Scientists have long searched for an economical and efficient way to convert methane into methanol, a more diverse carbon source that is an easily transportable liquid. It can be used to make thousands of products such as solvents, antifreezes and acrylic plastics; Fabrics and synthetic fibres. Adhesives, paints and chemicals used in medicine and agriculture.
Read also: A device with a thickness calculated by atoms. How did chemistry make electronics possible?
As oil reserves shrink, converting methane to soft fuels such as methanol becomes more attractive. A process developed by scientists in Manchester uses organic framework material and visible light to drive the conversion process.
To greatly simplify the process, when methane is exposed to a functional MOF containing iron monohydroxy sites, the activated oxygen molecules and energy from the light enhance the activation of the CH bond in methane to form methanol. This process is 100% selective – meaning there is no unwanted by-product – comparable to methane monooxygenase, which is an inherently enzyme for this process.the professor. Sihai Yang from the University of Manchester
It has been demonstrated that the solid catalyst can be isolated, washed, dried and reused for at least 10 cycles, or about 200 hours of reaction – without loss of yield. The new process is similar to how plants convert light energy into chemical energy during photosynthesis.
This process has been called the “holy grail of catalysis.” Instead of burning methane, it may now be possible to convert this gas directly into methanol, a fine chemical that can be used to make biofuels, solvents, pesticides and fuel additives in vehicles. This new MOF material may also be able to facilitate other types of chemical reactions by serving as a kind of test tube where we can combine different materials to see how they react.the professor. Martin Schroeder of the University of Manchester
By eliminating the need for high temperatures or pressures, and by using the energy of sunlight to drive the photo-oxidation process, the new conversion method can significantly reduce equipment and operating costs.

Echo Richards embodies a personality that is a delightful contradiction: a humble musicaholic who never brags about her expansive knowledge of both classic and contemporary tunes. Infuriatingly modest, one would never know from a mere conversation how deeply entrenched she is in the world of music. This passion seamlessly translates into her problem-solving skills, with Echo often drawing inspiration from melodies and rhythms. A voracious reader, she dives deep into literature, using stories to influence her own hardcore writing. Her spirited advocacy for alcohol isn’t about mere indulgence, but about celebrating life’s poignant moments.


![Matura 2022: Chemistry Extended. Some have already left the halls! why? [Relacja na żywo]](https://www.moviesonline.ca/wp-content/uploads/2022/05/1652690586_Matura-2022-Chemistry-Extended-Some-have-already-left-the-halls.jpg)