Amorphous graphite is a new type of solid material that can satisfy the growing appetite of carbonaceous materials. What can be useful?
Amorphous graphite was created by Scientists from Ohio University Under the supervision of physicist Prof. David Drabold and engineer Jason Tremblay. Details are described in Physical Review Letters.
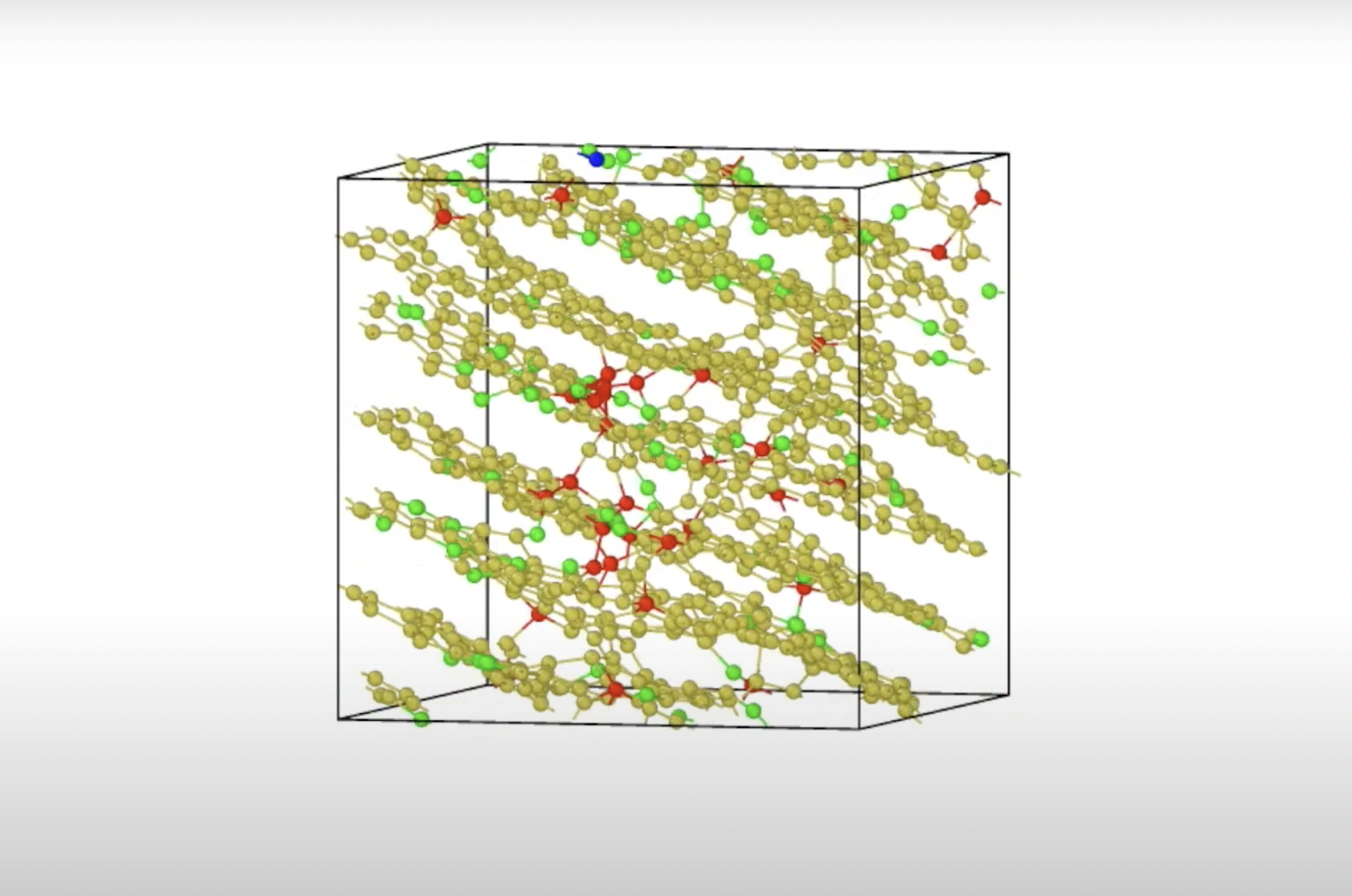
Graphite forms at very high temperatures (about 3000 K) and the individual layers are held together by electron gas. In “classic” graphite, the layers do not consist of complete hexagons (as in graphene). In the case of amorphous graphite, we are dealing not only with layers of hexagons, but also with pentagons and heptagons. This ‘turbulence’ reduces the electrical conductivity of the entire structure, but is still high in the hexagon-dominated regions.
In chemistry, the process of converting carbon materials into a layered graphite structure by high-temperature heat treatment is called graphite. From ab initio simulations and dynamic molecular simulations based on machine learning, we showed that pure carbon lattices have an overwhelming tendency to transform into a multi-layered structure in a window of significant density and temperature, layering even for random elemental configurations. The planar layers are amorphous graphite: three-coordinate carbon atoms that are not topologically arranged arranged in levels containing five, six, and heptacarbons.a. David Drabold
The scientists believe their achievement will stimulate experiments and research in the presence of amorphous graphite, which can be verified by exfoliation and/or experimental structural studies of the surface.
Since this phase is topologically disordered, only the usual “stacking log” of graphite is kept statistically. Layers without Van der Waals corrections for density function forces are observed, and we discuss the formation of a delocalized electron gas in the galleries (the space between planes) and show that interplanetary coherence is partly due to this low density electronegative gas. The in-plane electronic conductivity is significantly reduced compared to graphene.a. David Drabold
The work of scientists from Ohio University also heralds a new carbonaceous phase.
Carbon is a miracle element – you can make life out of it, diamond, graphite, Buckminster fullerene, nanotubes, graphene, and now that too. There’s a lot of basic physics interesting in it too – for example, how and why planes interconnect is surprising in itself for technical reasons.a. David Drabold

Echo Richards embodies a personality that is a delightful contradiction: a humble musicaholic who never brags about her expansive knowledge of both classic and contemporary tunes. Infuriatingly modest, one would never know from a mere conversation how deeply entrenched she is in the world of music. This passion seamlessly translates into her problem-solving skills, with Echo often drawing inspiration from melodies and rhythms. A voracious reader, she dives deep into literature, using stories to influence her own hardcore writing. Her spirited advocacy for alcohol isn’t about mere indulgence, but about celebrating life’s poignant moments.